ʻO ka wehewehe ʻana o In Vitro Diagnostic
ʻO ka In Vitro Diagnosis (IVD) e pili ana i ke ʻano diagnostic e loaʻa ai ka ʻike diagnostic clinical ma o ka hōʻiliʻili ʻana a me ka nānā ʻana i nā laʻana olaola, e like me ke koko, kohu, a i ʻole ka ʻiʻo, e ʻike, mālama, a pale i nā kūlana olakino.ʻO ka maʻi in vitro kahi kumu nui o ka maʻi lapaʻau, hiki ke hāʻawi i nā kuhikuhi kuhikuhi koʻikoʻi no nā hoʻolālā lapaʻau o nā kauka.He ʻāpana koʻikoʻi ʻo IVD o ka ʻōnaehana olakino e hōʻoia i ke olakino kanaka.
Mahele Makeke IVD
Ma muli o ka hoʻokaʻawale ʻana o nā kumu hoʻokolohua, hiki ke hoʻokaʻawale ʻia ka māhele mākeke IVD i Microbiology, Clinical Chemistry, Hematology, Coagulation, Immunoassay, Molecular Diagnostics, POCT, etc. nā reagents, nā mea hana, a me nā lawelawe.
ʻO ka ulu ʻana o ka IVD
Ka Papa 1:
ʻO ka hana ʻana o ka microscope i ala mai i kekahi mau ʻano hoʻokolohua kuʻuna.
Ka Papa 2:
ʻO ka hoʻomohala ʻana i ka lāʻau lapaʻau hou a me ka loaʻa ʻana o nā hopena enzyme-catalyzed a me nā hopena antigen-antibody i hoʻokumu i ke kumu no ka biochemical a me ka immunodiagnosis, no laila ke piʻi aʻe nei nā diagnostics in vitro a hoʻonui mālie i kēia manawa.
Papa 3:
ʻO ka hoʻohana ʻana o ka DNA double helix structure, monoclonal antibody technology, a me macromolecular markers technology i hāpai i ka hoʻomohala ʻana o nā diagnostics molecular in vitro diagnostic industry.
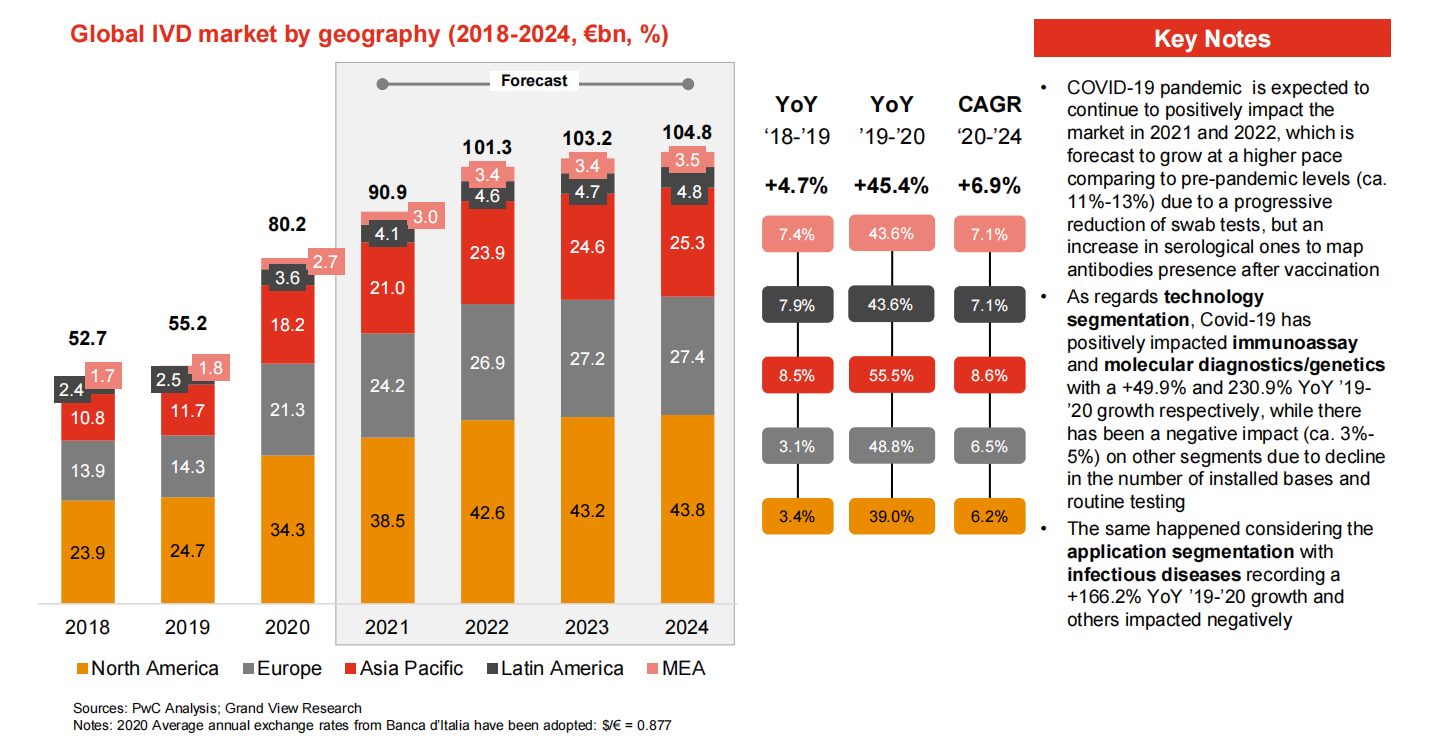
Makeke IVD honua
Ma luna o 70% o ka mākeke IVD honua e noho ʻia e ʻEulopa, ʻAmelika ʻAmelika a me Iapana.ʻEhā mau mea pāʻani honua nui ʻo Roche (Switzerland), Abbott (US), Thermo (US) a me Siemens (Kelemānia).Ua hui pū ʻia kēia mau ʻoihana ʻehā ma kahi o 51% ma 2017.



.png)





 Kāleka pāʻoihana
Kāleka pāʻoihana Pākē WeChat
Pākē WeChat English WeChat
English WeChat